| A Framework for Capturing Clinical Data Sets from Computerized Sources |
McDonald, C. J., Overhage, J. M., Dexter, P., Takesue, B. Y., Dwyer, D. M. |
Ann Intern Med |
|
http://www.annals.org/content/127/8_Part_2/675.long |
Variety in Data Reporting |
| Comparison of information content of structured and narrative text data sources on the example of medication intensification |
Turchin, A., Shubina, M., Breydo, E., Pendergrass, M. L., Einbinder, J. S. |
J Am Med Inform Assoc |
http://dx.doi.org/10.1197/jamia.M2777 |
|
EMR, chart notes |
| How to Document a Data Collection |
UC San Diego Supercomputer Center |
Online |
|
http://www.caida.org/data/how-to/how-to_document_data.xml |
Reproducible Research from online sources |
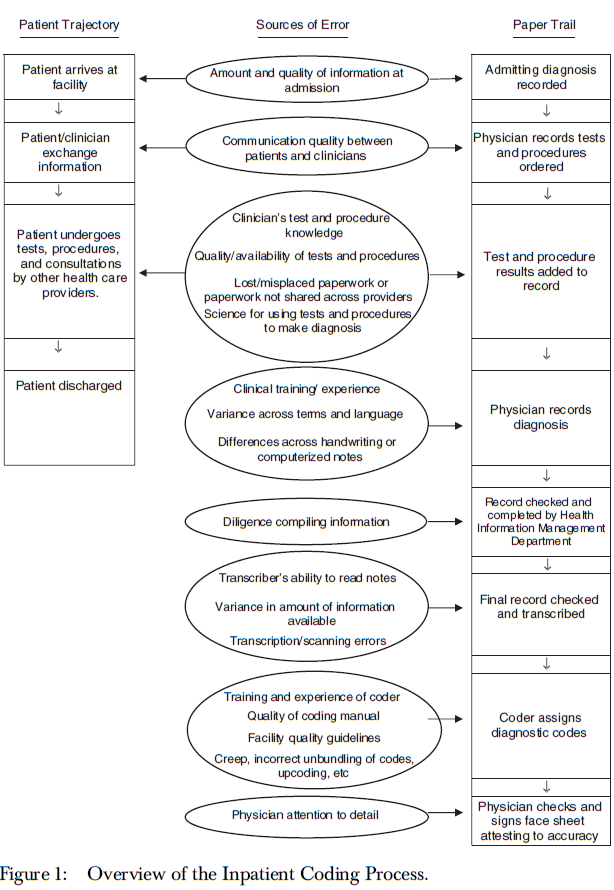
| Measuring diagnoses: ICD code accuracy |
O'Malley, K. J., Cook, K. F., Price, M. D., Wildes, K. R., Hurdle, J. F., Ashton, C. M. |
Health Serv Res |
|
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1361216/?tool=pubmed |
ICD 9: Sources of Error |
| Quality control in multicentric clinical trials. An experience of the EORTC Gynecological Cancer Cooperative Group |
Favalli, G., Vermorken, J. B., Vantongelen, K., Renard, J., Van Oosterom, A. T., Pecorelli, S. |
Eur J Cancer |
|
|
CT data |